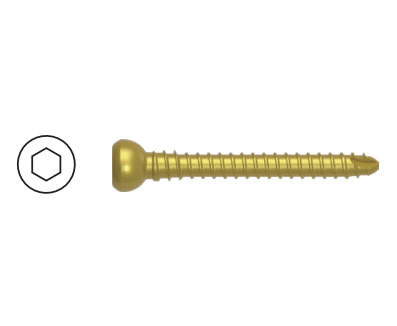
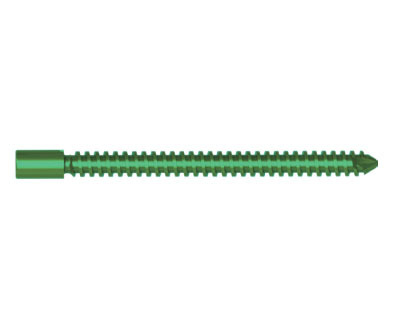
SF - I Multiaxial Pedicle Screw
The company established a comprehensive quality management system in accordance with ISO9001 and ISO13485 requirements in 2003, and has now obtained the Good Manufacturing Practice (GMP) certificate and CE certificate for implantable medical devices.
Category:
Product Details
| Product No. | Specification (mm) | Material | Remark |
| 22134530 | Ф4.5 × 30 | TA | |
| 22134535 | Ф4.5 × 35 | TA | |
| 22134540 | Ф4.5 × 40 | TA | |
| 22135035 | Ф5.0 × 35 | TA | |
| 22135040 | Ф5.0 × 40 | TA | |
| 22135045 | Ф5.0 × 45 | TA | |
| 22135530 | Ф5.5 × 30 | TA | |
| 22135535 | Ф5.5 × 35 | TA | |
| 22135540 | Ф5.5 × 40 | TA | |
| 22135545 | Ф5.5 × 45 | TA | |
| 22136035 | Ф6.0 × 35 | TA | |
| 22136040 | Ф6.0 × 40 | TA | |
| 22136045 | Ф6.0 × 45 | TA | |
| 22136050 | Ф6.0 × 50 | TA | |
| 22136535 | Ф6.5 × 35 | TA | |
| 22136540 | Ф6.5 × 40 | TA | |
| 22136545 | Ф6.5 × 45 | TA | |
| 22136550 | Ф6.5 × 50 | TA | |
| 22137035 | Ф7.0 × 35 | TA | |
| 22137040 | Ф7.0 × 40 | TA |
Keywords: SF - I Multiaxial Pedicle Screw
Inquiry
Please leave your contact information, our professionals will contact you as soon as possible!
Service Hotline
Tel:+86-519-86380098
Email:nanxiang@cznanxiang.cn
Address: No. 22, Changyang Road, Wujiang Economic Development Zone, Jiangsu Province
© 2025 Changzhou Nanxiang Medical Device Co., Ltd. ALL Rights Reserve. www.300.cn